Warm-Reactive Autoantibodies - Investigation: Alloadsorption:
If the patient has recently been transfused within the past 3 months or is too anemic to donate a sufficient volume of RBCs to allow an autoadsorption, then an alloadsoprtion using allogenic RBCs should be performed instead. The same principles as described for an autoadsorption applies to an alloadsorption: by selective removing autoantibodies from patient plasma, remaining alloantibodies can be identified.
To decrease the risk of alloantibodies being adsorbed along with the autoantibody, reagent RBCs used for alloadsorption must be pre-treated to remove as many antigenic binding sites as possible. This can be largely achieved using a combination of proteolytic enzymes which remove antigens such as M, N, S, s , Fya and Fyb, and the reducing agent dithiothreitol (DTT), which disrupts disulfide bonds and thereby destroys antigens within the Kell system.
This treatment will not remove Rh and Kidd system antigens, however, and two techniques are available to prevent these antigens from removing any clinically significant alloantibodies from the patient’s plasma, rendering them undetectable. The first is to select RBCs that have the same Rh and Kidd phenotype as the patient. If this is not possible (e.g., if the patient’s phenotype is unknown), a differential alloadsorption can be performed.
The principle of a differential alloadsorption is to use a variety of cells that, between them, will allow any common antibodies to the Rh and Kidd system to remain in patient plasma for identification. This can be achieved by selecting an R1R1, R2R2 and rr phenotype cell, and ensuring that one of the three is Jka homozygous and one is Jkb homozygous (see below).
Figure 1. Example of a uniform pan-reactive panel with a positive autocontrol suggesting the presence of a warm autoantibody.
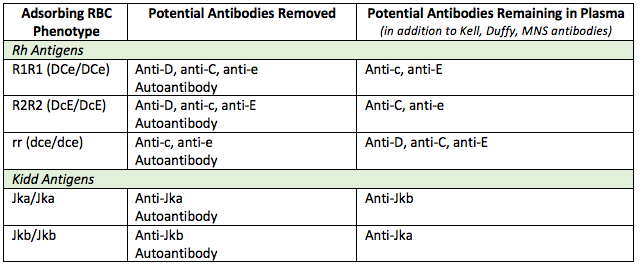
If, following the alloadsorption, the supernatant plasma from each of three cells used is non-reactive against a routine panel, then one can be confident that there are no common, clinically significant underlying alloantibodies. As noted, however, alloadsorptions cannot rule out the presence of an antibody to a high-frequency antigen, since such an antibody would also be adsorbed out of the patient’s plasma by any selected allogenic RBC.
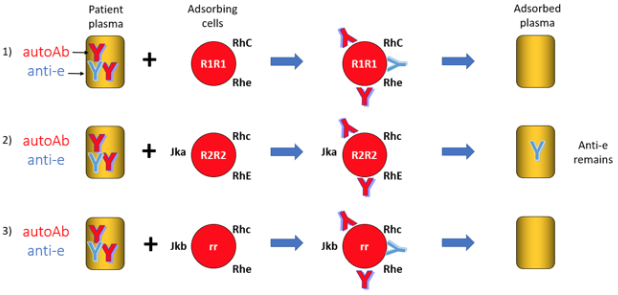
Figure 3. Example of a differential alloadsorption. The autoantibody will be removed by binding the treated auto-RBC. An alloanti-e antibody is left behind in the plasma adsorbed against R2R2 cells for identification against a routine antibody panel.
Next page: Reporting
